Methodology for Uni Knees
What is a Uni-Condylar Knee Replacement (UKR)?
There are certain patterns of knee arthritis where arthritis can develop within one of the three compartments of the knee. These “Compartments” are the medial (or inside), the lateral (or outside) and the patellofemoral compartments. Uni-condylar knee replacements (UKR) are used by surgeons to replace either the medial (inside) or lateral (outside) compartments. There are patellofemoral joint replacements for the patellofemoral joint
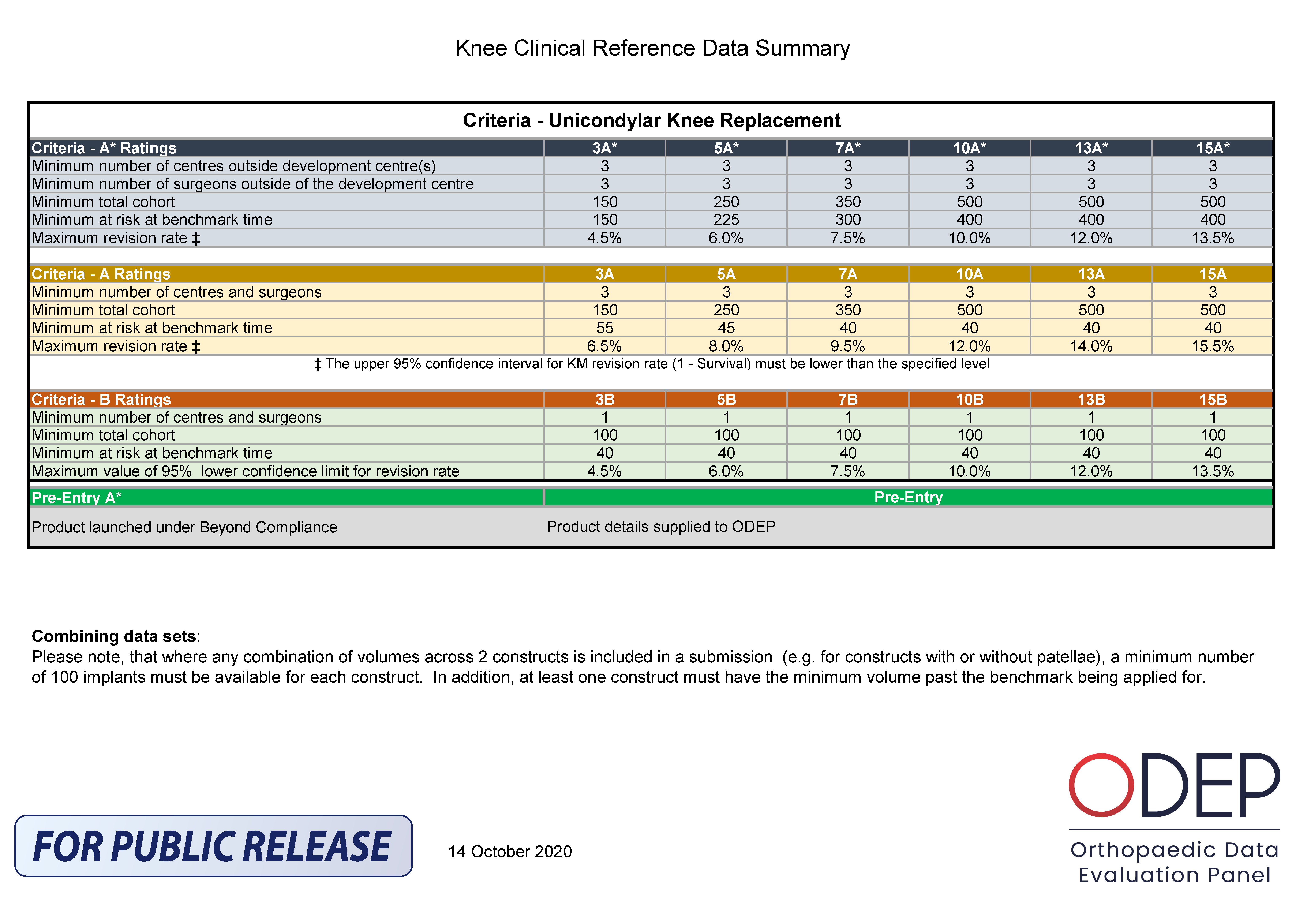
ODEP Methodology for Uni-Condylar Knee Replacement
The ODEP ratings were introduced for UKRs in 2016. A similar methodology has been used for UKR as for total knee replacements (TKR) – the implants are rated as an entire construct rather than for the individual parts. The benchmarks that are applied to all UKR constructs are demonstrated in Table 3.
The Uni-Condylar Knee Replacement Operation
Similar to TKRs, UKRs consist of a tibial component, a tibial insert and a femoral component. Patellofemoral replacements are different, involving a surface replacement of the trochlea of the knee and a resurfacing underneath the patella.
There are a number of perceived advantages of using UKR surgery over TKR surgery. They are well documented and they include a “smaller operation” with all its general health benefits and a shorter rehabilitation / convalescence period. The disadvantages , when compared to TKR surgery, is that the revision rates of UKR surgery have traditionally been higher.
Although there are fewer manufacturers with UKR brands in their portfolio than those who manufacture total knee replacements, they vary quite considerably from one to another. There are variances in the type of materials used, the fixation of the implants to the bone (cemented or uncemented) and the fixation of the polyethylene insert (either fixed or mobile bearing). The shapes, materials and fixation methods differ between brands depending on the brand philosophy.
As such there are a number of ODEP ratings available for each brand of UKR, depending the options that each brand offers.
Updated Benchmarks for UKR constructs
When ODEP started benchmarking UKRs in 2016 the published revision rates, data from worldwide registries were used to set the benchmarks for UKR. Since that time, outcomes from UKR have improved and this has been noted on worldwide registry data. As such, the benchmarks have been revised in 2020 to demonstrate this. Table 1 represents the updated benchmarks.
As with TKRs this methodology is intended:
• To encourage manufacturers to monitor all the options within one of their brands using the data available.
• To reduce the likelihood of “Big Data” camouflaging or obscuring a poorly performing subgroup within a brand.
As with all other implants, in submitting for an ODEP rating, to ensure objective consistency, the following guidelines should be observed. There are some specific caveats with respect to UCKRs which are noted below:
Manufacturers must fill in the ODEP submission form in its entirety. If they have any difficulty in accomplishing this, they should contact ODEP for advice.
Manufacturers will need to adhere to and certify that the following criteria are met:
• The implant must be manufactured by the said manufacturer unless clearly stated otherwise. The manufacturing process and the same standards are expected to be followed for the whole of the brand being submitted.
• The implant specifications are identical across the range, other than to accommodate increases in size.
• The material composition is identical across the range. If more than one type of plastic is used (regular, X-linked and additive) then separate submissions will be required.
• The implant surface finish is identical across the range.
• The locking mechanism for a modular insert must be consistent.
• The bone facing surface is identical in all sizes.
• Separate submissions are required for cemented, uncemented and hybrid options.
• 3D or surface layer additive technology manufacture will need separate submissions.
Ratings within brands
• ODEP will rate fixed and mobile bearing UKR implants separately.
• ODEP will rate cemented and uncemented UKR implants separately.
Bundling and Camouflage – No bundling options are currently offered for UKR variant constructs.
Data Sources
ODEP’s suggestions for data sources are as per all other implant categories, as mentioned on this website. ODEP expects that data sources used by manufacturers for their submissions should come from validate sources.

