Methodology for Shoulders
About ODEP Ratings
For detailed explanation, please refer to the ODEP Rating System page on this website.
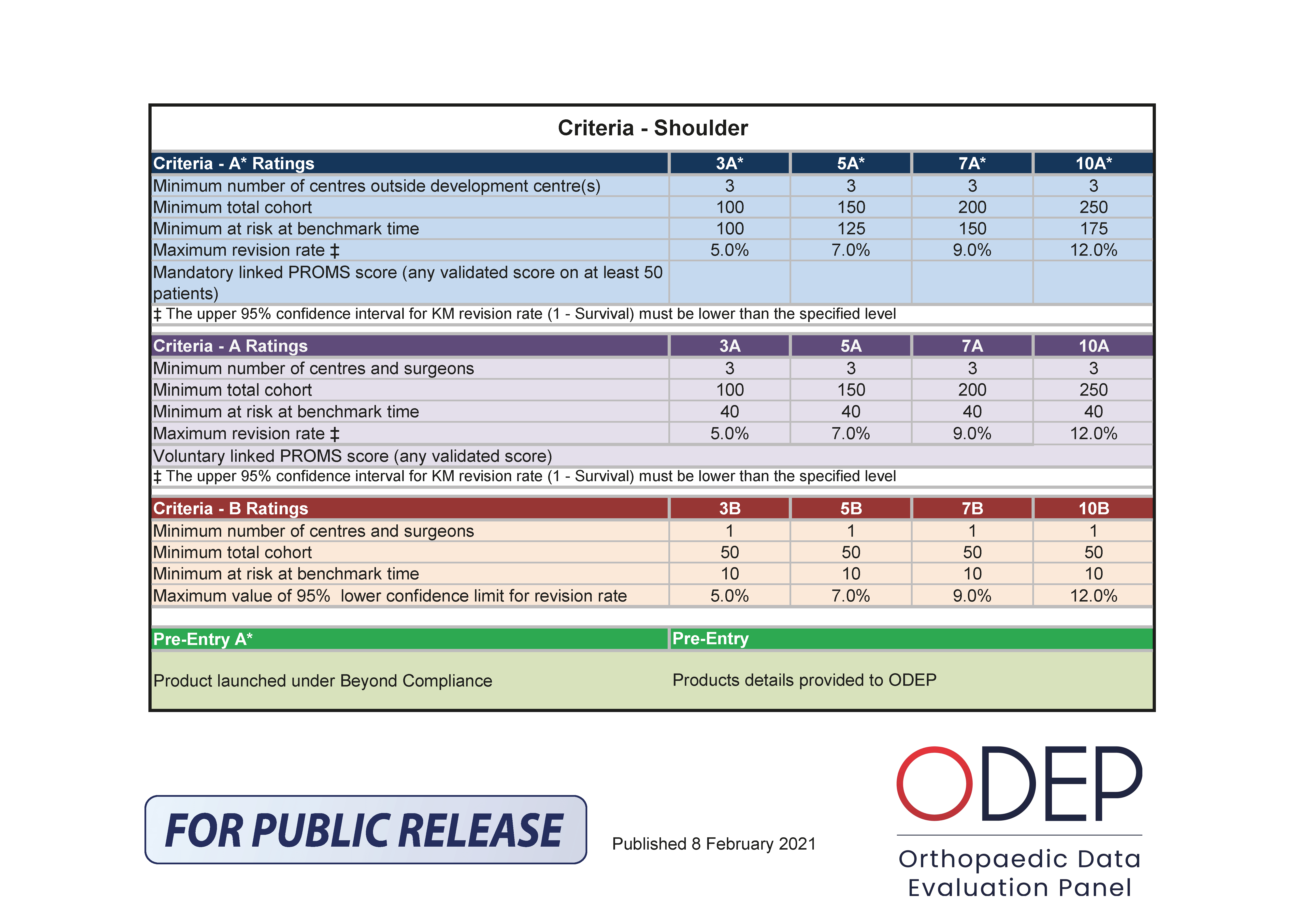
The letters “B”, “A” and “A*” are quantitative and qualitative benchmarks referring to the strength of evidence provided by the manufacturer.
A – Strong evidence – generally higher numbers of patients (giving greater confidence in the results presented), with all patients being subject to follow-up (their outcomes recorded)
B – Acceptable evidence – means that the submission includes smaller numbers of patients or centres than the A rating (giving less confidence in the results than A), but sufficient data to demonstrate compliance
When the number of surgeons / centres is greater than 3 and the number of patients past the benchmark is greater than the minimum number required for an A rating, B ratings cannot be offered.
Considering the data used, Registry data typically carry more weight than a peer reviewed publication, which in turn would carry more weight than an in-house manufacturer-initiated study. In 2023, ODEP implemented a new guideline requiring manufacturers to ensure that any in-house data used for ODEP submissions complies with ISO 14155:2020 standards. This provides ODEP with extra reassurance about the quality of the submitted data.
Background
It was in 2015 that members of BESS (British Shoulder and Elbow Society) decided that they would like to develop ODEP for Shoulders. So a group of enthusiastic shoulder surgeons got together to develop the benchmarks and the submission form.
It was decided to introduce ODEP for “Reverse TSR” to start with. A “dummy run” was instituted and the first benchmarks were awarded in 2017.
After successfully introducing ODEP for Reverse TSR, glenoid implants, Anatomic and TSR for trauma followed. They were all introduced after a successful “Dummy Run”.
Shoulder replacement is a major operation which involves replacing the native shoulder joint. This can be performed either by a standard “anatomic” implant replacing the head, if indicated combined with a device replacing the glenoid, or by a reversed arthroplasty, where the head of the humerus is replaced by a humeral “socket” and the glenoid part consist of a “ball”, the “glenosphere”. In most standard anatomic humeral prostheses the head of the humerus is excised and a stem passed down the inside of the proximal humerus on to which a head, or a socket, in case of a reversed arthroplasty, is attached via a taper / trunnion system. In a resurfacing procedure the humeral head is left in situ and it is resurfaced. Fixation of all these implants is either by using a bone cement (“Cemented”) or by their bone facing surface attracting and becoming incorporated in the local host bone (“Uncemented”).
Evolution
Since the formation of the ODEP Shoulder arthroplasty panel in 2017 there has been a sustained growth of shoulder arthroplasty procedures. The UK National Joint Registry (NJR) introduced shoulder arthroplasty procedures in 2012 and demonstrated significant increases (> 25-30% growth annually) in implantation between 2012 and 2015, followed by steady growth in the last years. As time has gone by the system has developed and taken on much of the format used by hips and knees.
Benchmarking shoulder implants produces different challenges from knee and hip implants. One of the biggest challenges is appraising a shoulder replacement with poor functional results, but where a revision has not taken place. In 2021, it was therefore decided to include PROMs data and reward these outcomes with a * (Star) rating. The number of Star ratings is growing, but the NJR PROMS data is still very limited.
Another challenge is that only a few registries (the NJR and the registries in the Netherlands, Australia, New Zealand, Finland, being the exceptions) generate shoulder data. NJR shoulder data goes back to 2012, but year on year since, has increased in volume.
ODEP Shoulders works closely with NJR and BESS. The final NJR classification of shoulder implants, the NJR Minimum Data Set (MDS) and the NJR Implant Scrutiny Committee take note of ODEP Shoulders suggestions and concerns.
Shoulder ratings
ODEP’s evolving Shoulder policy of rating stems, cups, reversed implants resurfacing implants and trauma implants separately is for several reasons. Given the current volume and variety of implants all separate and interchangeable parts request separate submissions, as do augmented versions or separate augments.
General Assumptions in Rating
1. As with Hips and Knees the panel is concerned about “camouflage”, particularly with respect to the glenoid and the various augments currently on the market. Therefore, the Panel aims for a high granularity, although it realises that sometime pragmatic choices have to be made.
2. Some manufacturers, albeit a small number, only manufacture either cups or stems.
3. As many anatomic stems are also in use as hemiarthroplasty, separate rating for stem and glenoid in the anatomic prosthesis is logical.
4. As some manufacturers produce stems with a design or coating specially for the treatment of proximal humeral factures, separate rating for trauma implants is required.
5. Resurfacing implants have definitely different aspects of fixation
6. As reversed arthroplasty can only function with a stem and glenosphere construct that is specifically designed, these are rated as one construct (stem + metaphysis + humeral socket + liner + glenosphere + baseplate)
6. Separate submissions are required for cemented, uncemented and hybrid options.
Rating Stems
An ODEP shoulders rating will apply to a stem brand where each stem satisfies the following stipulations:
• It is used as an anatomic shoulder replacement or as a hemiarthroplasty
• The material composition is identical across the range
• The implant surface finish is identical across the range
• The taper/trunnion design is identical across the range
• Implant specifications are identical across the range, other than to accommodate increases in size of the manufacturer’s “Standard” stem width and offset
Stems will be considered as a separate group and need a separate submission when:
• Implants are marketed as being “Short stem”
• Implants are marketed as “Long Stem”
• Stems that can be adapted from anatomic to reversed need to be rated separately when used in a reversed construct.
Rating Glenoid Implants
An ODEP rating will apply to a glenoid brand where each glenoid satisfies the following stipulations:
• It is used as a primary shoulder arthroplasty
• The material from which it is made is identical across the range
• The bone facing surface finish/coating is identical across the range
• The implant specifications are the same across the range (including modular liner locking mechanism), other than to accommodate incremental increases in cup size and screw holes.
Glenoid implants will be considered as a separate group and need a separate submission:
• when designed with bone defect augmentations or additions
Rating Trauma stems
An ODEP rating will apply to trauma arthroplasty brand where each construct satisfies the following stipulations:
• It is to be used as a shoulder replacement in fracture cases
• The material composition is identical across the range
• The implant surface finish is identical across the range
• The taper/trunnion design is identical across the range
• Implant specifications are identical across the range, other than to accommodate increases in size of the manufacturer’s “Standard” stem width and offset
Trauma stems will be considered as a separate group and need a separate submission:
• When Implants are marketed as “Long Stem”
• Stems that can be adapted from anatomic to reversed need to be rated separately when used in a reversed construct.
• The revision rate considered for rating anatomic trauma stems is “All failure modes – any component revised”.
Rating resurfacing implants
An ODEP rating will apply to resurfacing brand where each construct satisfies the following stipulations:
• It is to be used as a resurfacing shoulder replacement
• The material composition is identical across the range
• The implant surface finish is identical across the range
• The bone facing surface finish/coating is identical across the range
• Implant specifications are identical across the range, other than to accommodate increases in size of the manufacturer’s “Standard” stem width and offset
Rating Reversed arthroplasty constructs (stem + metaphysis + humeral socket + liner + glenosphere + baseplate)
An ODEP rating will apply to reversed arthroplasty brand where each construct satisfies the following stipulations:
For the stem:
• It is used as primary reversed shoulder arthroplasty
• The material from which it is made is identical across the range
• The bone facing surface finish/coating is identical across the range
• The implant specifications are the same across the range (including modular liner locking mechanism), other than to accommodate incremental increases in cup size and screw holes.
• The taper/trunnion design is identical across the range
For the humeral socket:
• The material composition is identical across the range.
(If more than one type of plastic is used then separate submissions are required).
• The bone facing surface finish/coating is identical across the range
• The implant specifications are the same across the range (including modular liner locking mechanism), other than to accommodate incremental increases in size
• The taper/trunnion design is identical across the range
For the liner
• The material composition is identical across the range. If more than one type of plastic is used (regular, X linked and additive) then separate submissions are required.*
For the baseplate
• The material composition is identical across the range.
(If more than one type of plastic is used then separate submissions are required).
• The bone facing surface finish/coating is identical across the range
• The implant specifications are the same across the range (including modular liner locking mechanism), other than to accommodate incremental increases in size and screw holes.
• The taper/trunnion design is identical across the range
For the Glenospheres (reversed glenoid)
• The material from which it is made is identical across the range
• The implant specifications are the same across the range
• Glenoid components will be considered as a separate group and need a separate submission when bone defect expansions or additions are added.

