Methodology for Spine Cervical Cage
ODEP Spine Webinar – 2025
In July 2025, the Spine Panel hosted a highly successful webinar. The event was well-attended and provided manufacturers and distributors of spinal implants with valuable insights into the current status of the ODEP submissions process for cervical cages.
You can access the recording of the webinar on the UKSSB website under the news section –
https://www.ukssb.com/post/odep-bc-spine-webinar-broadcast-on-thursday-3rd-july-2025-via-zoom-webinars
and also on YouTube –
https://www.youtube.com/watch?v=1GUEDc0Ot9Y
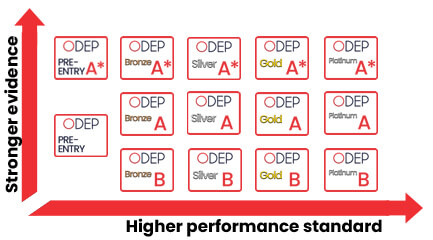
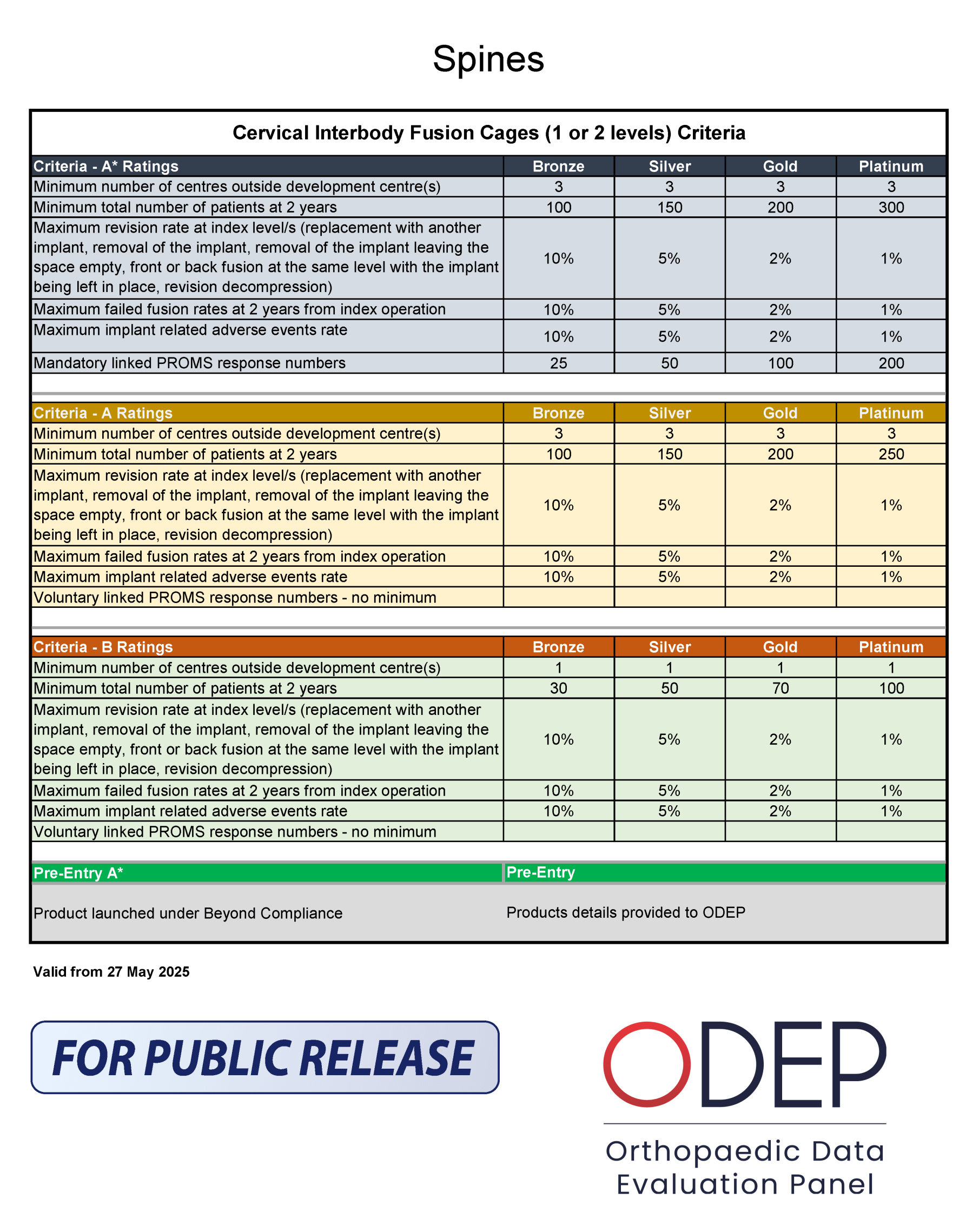
ODEP Criteria Grid
The grid outlines ODEP criteria for cervical interbody fusion cages across different rating levels: A*, A, and B. Each rating level is further divided into Bronze, Silver, Gold, and Platinum categories.
The data for all 12 benchmarks is evaluated at 2 years.
The standard validity period for all ratings is 3 years, with an additional one year of grace period. However, the Panel may reduce the rating validity period, in which case the standard submissions review feedback to the manufacturer will clearly state so. Where a reduced rating validity period is confirmed, the year of grace does not apply.
The ODEP rule for mobile devices, where progression is expected for each consecutive submissions (e.g. 3A – 3A* – 5A* – 7A, etc), does not apply to fixed devices.
Rating Levels (A*, A, B):
- A*: Represents the highest level of evidence and performance criteria.
- A: Indicates strong evidence but slightly less stringent than A*.
- B: Represents initial or lower levels of evidence and performance criteria, this is aimed at “boutique” devices, where the number of centres / surgeons is limited to just one (but excludes the designer surgeons for this device).
Bronze, Silver, Gold, Platinum Labels:
These labels indicate the tier of performance and evidence within each rating level. Higher tiers (Gold, Platinum) require more stringent criteria and higher performance standards.
- Bronze: Basic level of performance and evidence.
- Silver: Intermediate level.
- Gold: Advanced level.
- Platinum: Highest level of performance and evidence.
Interpretation of Labels and Criteria
The Bronze, Silver, Gold, Platinum labels indicate increasing levels of evidence and performance standards rather than just the number of implanted devices or sales. The criteria focus on:
- Number of patients: Higher tiers require more patients, indicating more robust evidence.
- Revision rates: Lower rates in higher tiers suggest better performance and reliability.
- Failed fusion rates: Lower rates in higher tiers indicate higher success rates.
- Adverse events rates: Lower rates in higher tiers imply better safety profiles.
- PROMs response numbers: Higher numbers in higher tiers reflect more comprehensive patient-reported outcomes.
Conclusion
The labels represent a hierarchical structure of evidence and performance, with higher tiers requiring more stringent criteria and demonstrating better outcomes. This methodology ensures that devices are evaluated rigorously, promoting high standards in patient care and device performance.